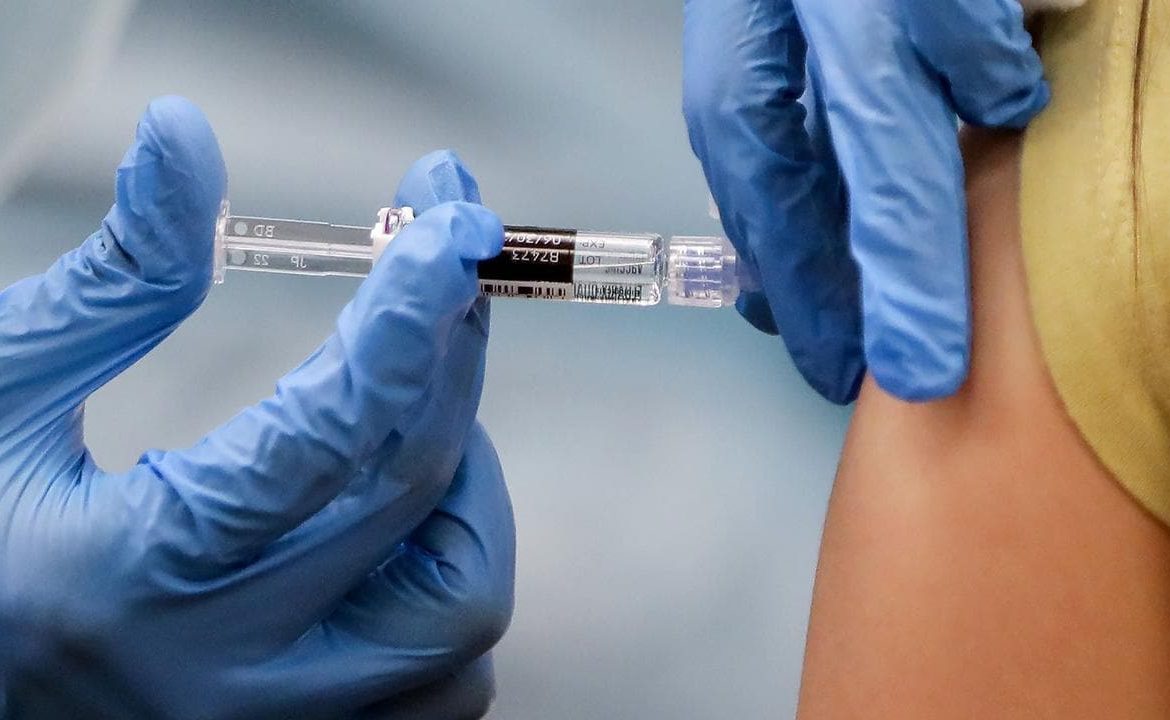
Ordinary Americans could begin receiving a coronavirus vaccine within the “first four months” of 2021 in a move that has been described as a “game changer”.
The good news was confirmed by the US government’s top infectious diseases expert, Dr Anthony Fauci, who is leading the coronavirus fight in America.
Speaking on CNN today, Dr Fauci said he expected low-risk Americans would begin receiving the jab by April next year, with those deemed to be at a higher risk getting it earlier.
Dr Fauci told host Jake Tapper the breakthrough was “extraordinary” and that he expected people’s willingness to get the jab would be boosted by the vaccine’s efficacy.
Dr. Anthony Fauci says COVID vaccine likely available to average citizens without conditions by next April. Calls news of its effectiveness “extraordinary” Speaking on CNN says “challenges” in distribution can be overcome. #7News pic.twitter.com/Hlou1hTHkK
— Dan Hausle (@dhausleon7) November 10, 2020
“If you hear something is 90 to 95 per cent effective, you’re more likely to get vaccinated,” Dr Fauci told CNN.
His comments come as the head of one of the companies that announced promising results for a coronavirus vaccine refused to say whether or not the vaccine in question will deliver on the world’s high hopes of a return to normal.
In an interview with the BBC overnight, Sean Marett, BioNTech’s Chief Business and Commercial Officer, revealed that the German pharma giant, along with partner Pfizer, made a decision to make vaccine development a priority at the end of January, shortly after the sequence of the virus was published.
Hugely promising results from their coronavirus vaccine trial has fuelled optimism around the world that humanity may be a step closer to defeating the worst pandemic in a century, but Marett was hesitant to confirm it as a miracle cure just yet, despite Pfizer and BioNTech announcing that their vaccine candidate was 90 per cent effective in preventing COVID-19.
“We are a significant step closer to providing people around the world with a much-needed breakthrough to help bring an end to this global health crisis,” Pfizer chairman Albert Bourla said.
RELATED: UK task force chair warns we might never have vaccine
RELATED: Australian shares rally following promising vaccine news
RELATED: Health Minister reveals Australia’s plan to manufacture vaccines
UPDATE: We are proud to announce, along with @BioNTech_Group, that our mRNA-based #vaccine candidate has, at an interim analysis, demonstrated initial evidence of efficacy against #COVID19 in participants without prior evidence of SARS-CoV-2 infection.
— Pfizer Inc. (@pfizer) November 9, 2020
A vaccine is seen as the best hope to break the cycle of deadly virus surges and severe restrictions across much of the world since COVID-19 first emerged in China late last year and unleashed devastation on the global economy.
Pfizer and BioNTech’s candidate is one of more than 40 versions in development, but no other has yet made similar claims about effectiveness.
“We saw the disease as something that would affect our lives and we had technology that we believe could work,” said Marett.
“We have a technology that’s very flexible and we really focused the company on the program. It’s the technology that allows us to do things quickly from drawing board to testing in humans.”
We welcome the encouraging vaccine news from @pfizer & @BioNTech_Group & salute all scientists & partners around the 🌍 who are developing new safe, efficacious tools to beat #COVID19. The 🌍 is experiencing unprecedented scientific innovation & collaboration to end the pandemic!
— Tedros Adhanom Ghebreyesus (@DrTedros) November 9, 2020
There are 3 critical areas where we must demonstrate success before filing for EUA of our #COVID19 vaccine
▶️ Evidence of efficacy in most vaccinated patients
▶️ Evidence of safety w/ data from thousands of patients
▶️ Manufactured consistently at the highest quality standards
— Pfizer Inc. (@pfizer) November 9, 2020
Mr Marett revealed it took three months for an appropriate vaccine candidate to be trialled on humans and that the vaccine had been tested on the elderly, who are among the most vulnerable to the virus.
He said the intention is to “cover the complete adult population and that’s how we’ve designed our clinical trial”.
The scientific community reacted positively, with top US expert Anthony Fauci describing the results as “extraordinary” and World Health Organisation boss Tedros Adhanom Ghebreyesus hailing the news as “encouraging”.
The companies said they could pass the final hurdles for a US rollout later this month, and could supply up to 50 million doses globally this year and up to 1.3 billion next year.
But others pointed out that data from the ongoing trial was still needed for review, including the ages of the participants.
Successful vaccines require international approval before they can be manufactured and distributed. Effectiveness, of which the trial currently claims, is only one of three requirements needed to apply. Pfizer claims it will meet the next two – safety and manufacturing standards – by “the third week of November”.
Mr Marett refused to answer on whether the trial will “deliver on such high hopes” of a return to normal, but said the desire is to “get everyone back to as far as they can, the way they lived before”.
But, he said, “we believe that we can really achieve this”.
The Pfizer candidate uses the “messenger RNA” method to fight the virus, but a major disadvantage of such vaccines is that they need to be stored in specialist deep freezers as opposed to refrigerator-level temperatures required for some other types.
Experts have also raised concerns about the capacity of poorer nations, especially in warm climates, to set up safe supply chains for such a vaccine.
Wall Street and European markets soared after the announcement, and Asian markets continued the rally on Tuesday, with worst-hit sectors such as airlines, travel, entertainment and energy getting a boost.
Yet ABC’s senior business correspondent Peter Ryan warned economic recovery is a long way off.
“We shouldn’t be expecting any immediate snapback, the spread of the virus is worsening in the United States and Europe, which could see more lockdowns,” he said.
Meanwhile, Brazil’s health regulator announced Monday it had suspended clinical trials of a Chinese-developed vaccine — one of the most advanced candidates — after an “adverse incident” involving a volunteer recipient.
Its maker Sinovac said it was confident in the safety of the vaccine.
Let us hope that Pfizer are right about their vaccine. If not then more lockdowns.
— Nigel Farage (@Nigel_Farage) November 9, 2020
‘A VERY DARK WINTER’
The coronavirus has infected close to 51 million people worldwide, with more than 1.2 million deaths.
The United States remains the hardest-hit nation at more than 10 million cases and nearly 240,000 deaths, and the pandemic was one of the top issues for voters in the presidential election this month.
Joe Biden, who had slammed President Donald Trump’s handling of the crisis, spared no time in announcing a COVID-19 task force after he was declared the winner of the election.
“We’re still facing a very dark winter,” he said.
“The bottom line: I will spare no effort to turn this pandemic around once we’re sworn in.” Trump had clashed repeatedly with his own government experts, often refusing to back restrictions or even wear a mask in public. After the Pfizer announcement, he claimed — without evidence — that the news was delayed until after the election to damage him.
There was a separate breakthrough when the US Food and Drug Administration on Monday granted emergency approval to a synthetic antibody treatment for COVID-19 developed by the pharma company Eli Lilly.
Bamlanivimab, which was shown to reduce the risk of hospitalisation and emergency room visits, is the first major drug to be approved that was designed specifically for the coronavirus.
‘OUT OF CONTROL’
The vaccine news will be of particular relief to people in Europe — the current focal point of the pandemic and the region subject to the most widespread restrictions.
Italy was edging closer to a full lockdown, with experts warning of pressure on hospitals.
“There is no doubt that the situation is largely out of control,” said Massimo Galli, head of the infectious diseases department at Milan’s Sacco hospital.
Grim news kept coming elsewhere, with Russia surpassing its record for daily infections again Monday.
Hungary is now one of the hardest-hit countries in terms of deaths proportionate to its population, and the government announced new national restrictions to come into force Wednesday.
Portugal meanwhile entered a state of emergency that will see curfews imposed on most of the population.

— with AFP
Originally published as COVID vaccine deployed ‘within months’