“The analysis shows no serious safety concerns,” the statement said, without detailing whether any participant suffered side effects.
It wasn’t immediately clear if the announced results included only those taking part in the testing in the UAE or if they also include results from China and elsewhere. The statement described the vaccine as receiving “official registration” without elaborating on what that meant.
Emirati officials and Sinopharm did not respond to questions from The Associated Press.
However, calls on Wednesday to SEHA, the Abu Dhabi health authority, included an option for individuals to schedule an appointment to receive a “COVID-19 vaccine”. The centre was swamped with calls following the announcement on the vaccine’s efficacy.
The Sinopharm vaccine has been approved for emergency use in a few countries and the company is still conducting late-stage clinical trials in 10 countries. Morocco is gearing up for an ambitious COVID-19 vaccination program, aiming to vaccinate 80 per cent of its adults in an operation starting this month that’s relying initially on the Sinopharm vaccine.
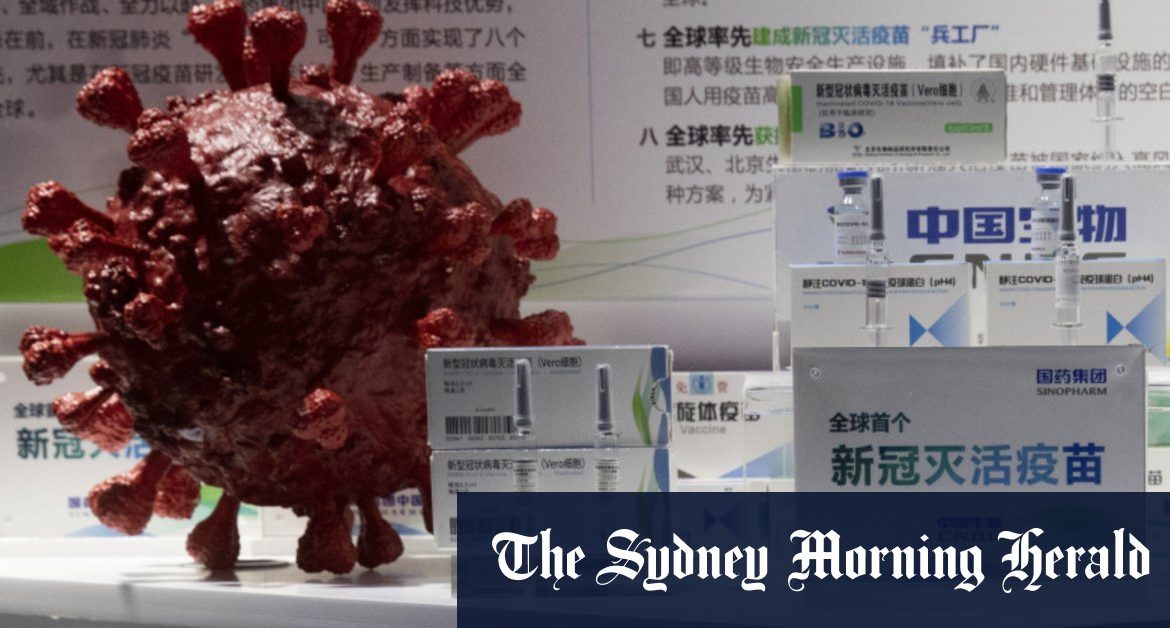
Sinopharm’s shot relies on a tested technology, using a killed virus to deliver the vaccine, similar to how polio immunisations are made. Leading Western competitors, like the shot made by Pfizer and its German partner BioNTech, use newer, less-proven technology to target the coronavirus’ spike protein using RNA. The United Kingdom already has begun vaccinating people with the Pfizer shot.
Top officials in the UAE, including Dubai’s ruler, Sheikh Mohammed bin Rashid Al Maktoum, have publicly received the shots as part of the vaccine testing.
Already, Pfizer reports its shot as being 95 per cent effective, while another RNA candidate from Moderna appears to be 94.5 per cent effective. Results suggest a third vaccine from Oxford University and AstraZeneca is safe and about 70 per cent effective, but questions remain about how well it may help protect those over 55.
In transport, the Pfizer and Moderna vaccines must remain at cold temperatures – negative 70 degrees Celsius and negative 20 degrees Celsius, respectively.
The Sinopharm vaccine is believed to be able to travel at a temperature of 2 to 8 degrees Celsius, a major draw for areas of the world where nearly 3 billion people live without stable electricity and refrigeration. In November, the Communist Party secretary for Sinopharm said almost 1 million people in China had received its vaccine.
Meanwhile on Wednesday, Abu Dhabi announced it would resume “all economic, tourism, cultural and entertainment activities in the emirate within two weeks”. It attributed the decision to “successes” in halting the spread of the coronavirus.
Abu Dhabi has required those travelling into the emirate from the UAE’s other six sheikhdoms to take a coronavirus test within 48 hours prior to arrival.
Since the pandemic began, the UAE has detected more than 178,000 cases of the virus, with 160,000 recoveries and nearly 600 deaths. Its aggressive testing policy has seen over 17 million tests conducted in a country home to just over 9 million people.
Loading
In Iran, meanwhile, President Hassan Rouhani angrily blamed US sanctions for making it difficult for Tehran to purchase medicine and health supplies amid the pandemic.
And in Israel, Prime Minister Benjamin Netanyahu welcomed the first shipment of doses of the Pfizer vaccine, calling it a “big day of celebration” for the country.
“I believe in this vaccine. I expect it will receive the appropriate approval in the coming days,” Netanyahu said, adding that he intended to be the first to receive it. “We see the end of the pandemic.”
AP
Get our Coronavirus Update newsletter
Stay across the news you need to know related to the pandemic. Sent Monday and Thursday. Sign up here.
Most Viewed in World
Loading